In the ever-evolving world of energy technologies, bipolar membranes play a crucial role in the development of various applications such as electrolyzers and hydrogen fuel cells. While these membranes have been utilized for over six decades, there is still much to learn about their underlying working principles and ion solvation kinetics. The key to enhancing the efficiency and performance of these technologies lies in gaining a better understanding of how bipolar membranes function and how they can be optimized for different applications.
A recent study conducted by researchers at the Fritz-Haber Institute of the Max Planck Society delved into the water dissociation and ion solvation kinetics at the interface of bipolar membranes. By examining these fundamental principles, the researchers were able to gather valuable insights that could shape the future design of bipolar membranes and electrocatalysts for fuel cells. The study, published in Nature Energy, sheds light on the intricate processes that occur within these membranes and how they impact the overall efficiency of energy technologies.
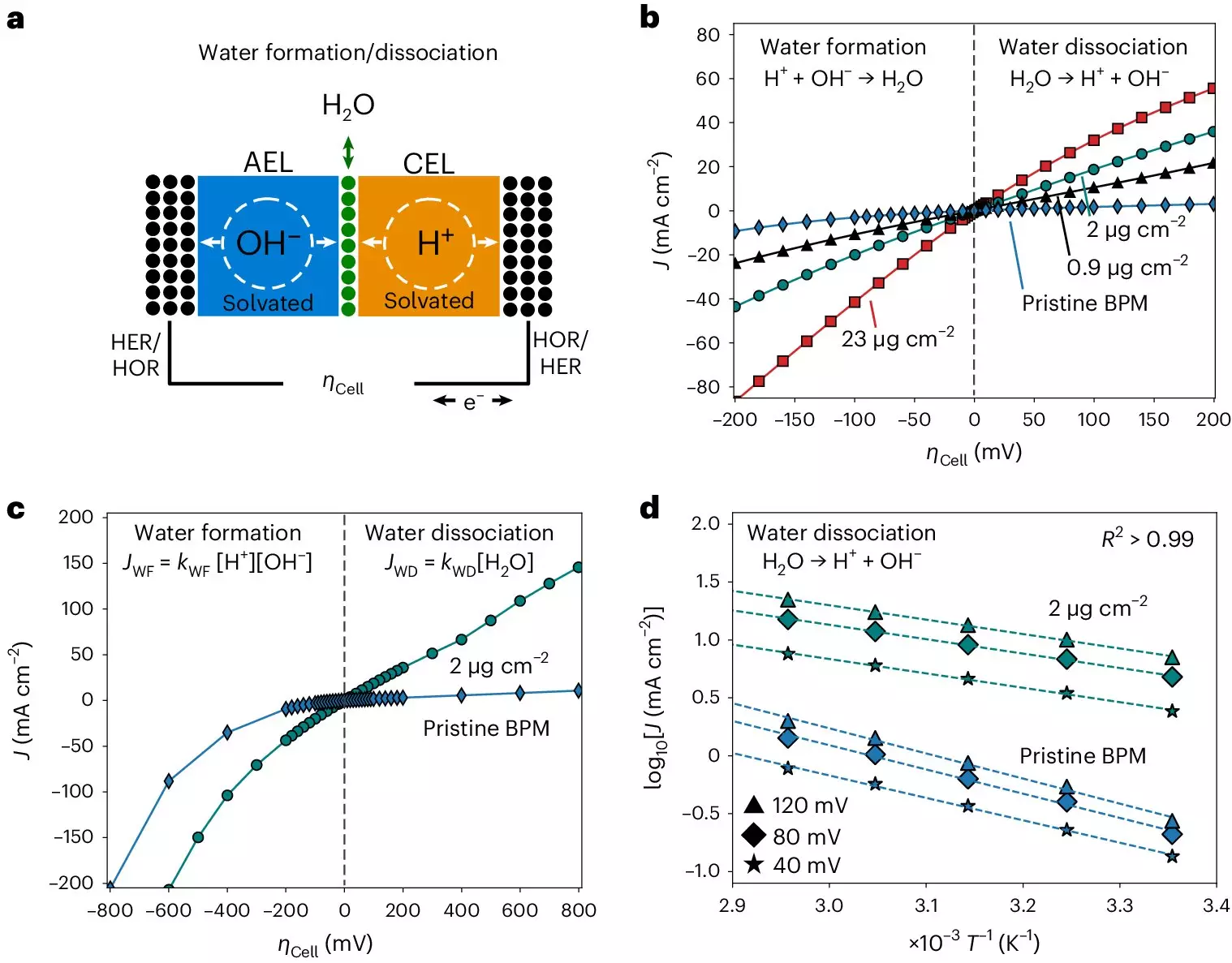
In order to carry out their study, the research team faced various challenges in setting up an experimental system that would allow them to study the kinetics of bipolar membranes accurately. The need to prevent undesirable interferences from the crossover of electrolyte ions required a system that could apply continuous physical pressure on the membrane electrode assembly. Additionally, the researchers had to design the system to control variables such as temperature and humidified gases to conduct in-depth analysis. Overcoming these challenges was essential in obtaining precise and reliable measurements for their study.
The measurements collected by the researchers revealed bias-dependent relationships between activation entropy and enthalpy within bipolar membranes. These findings suggest a connection between interfacial capacitance dispersion and the functioning of the membranes under different biases. The team also observed that solvation kinetics in bipolar membranes exhibit unique characteristics that are independent of the catalysts used, pointing to entropic changes in the interfacial electrolyte as a key factor. Understanding these insights can pave the way for the development of more efficient bipolar membranes for various applications, such as electrodialysis and CO2 electrolyzers.
The research conducted by the Interface Science Department of the Fritz Haber Institute highlights the significance of entropic changes at liquid-solid interfaces in energy technologies. The findings not only contribute to the design of better performing electrocatalysts for specific chemical reactions but also pave the way for the development of green hydrogen generation from alkaline electrolytes. The researchers are focused on addressing fundamental questions related to water formation reactions in bipolar membranes and exploring collaborations for applications in fuel cells and electrodialysis systems. As the field of ion solvation in electrocatalysis continues to progress, the team is working on multiple projects to further understand and optimize these processes for enhanced energy technologies.
The study conducted by researchers sheds light on the complex mechanisms at play within bipolar membranes and their impact on energy technologies. By unraveling the fundamental principles governing the functioning of these membranes, researchers can pave the way for more efficient and sustainable energy solutions in the future. The insights gained from this study can inform the design and optimization of bipolar membranes and electrocatalysts, ultimately leading to advancements in various energy applications.
Leave a Reply