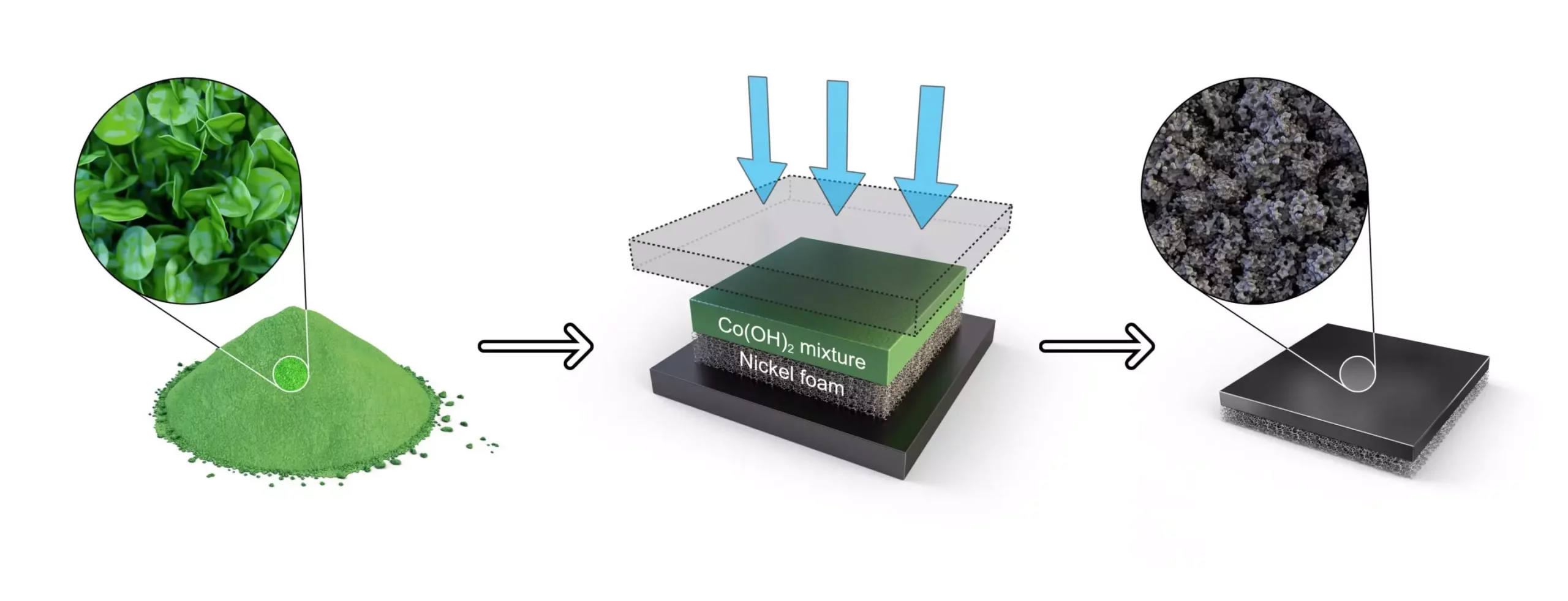
A groundbreaking research conducted by Dr. Sung Mook Choi and his team at the Korea Institute of Materials Science has led to the development of a one-step electrode fabrication process in South Korea. This innovative process, recently published in the journal Applied Energy, revolutionizes the production of electrodes for anion exchange membrane water electrolysis by directly converting raw materials into electrodes at a mass-producible level. By utilizing a one-step hot-pressing process, cobalt hydroxide is transformed into a cobalt oxide catalyst, resulting in the formation of a highly uniform catalyst layer. This new method simplifies the existing complex process by eliminating multiple steps such as hydroxide-oxide-crushing-dispersion-coating-drying, and replacing them with a single coating step. The team has achieved a 60% reduction in the manufacturing process compared to the traditional method, while ensuring the creation of a uniform catalyst layer and significantly improving the efficiency of hydrogen production and durability during continuous operation.
Optimization of the Fabrication Process
In order to optimize the one-step electrode fabrication process, the research team meticulously controlled the conditions of the cobalt hydroxide slurry, as well as the temperature and pressure during the hot-pressing process. This careful control resulted in the successful fabrication of a catalyst layer composed of uniform oxide particles, each measuring 10 nanometers in size. The team proceeded to use the developed electrode to create a membrane electrode assembly (MEA) incorporating an anion exchange membrane and a hydrogen evolution catalyst. The stability and performance of this assembly were confirmed in a commercial-scale water electrolysis cell, showcasing the potential for stable anion exchange membrane water electrolysis with high hydrogen generation efficiency and a low degradation rate.
Future Prospects
The development of this new technology opens up exciting possibilities for the future of anion exchange membrane water electrolysis. It is estimated that by 2030, green hydrogen production from water electrolysis could reach a staggering 11 million tons and 69 gigawatts of capacity. In Korea, efforts are already underway to demonstrate a megawatt-level anion exchange membrane water electrolysis system by 2024, with the goal of commercializing such a system by 2030. Despite Korea’s current technological standing at approximately 70-80% of world-class standards in this field, there is a pressing need for increased investment and the localization of key technology. By securing the source technology for mass-produced electrode manufacturing, Korea aims to gain a global competitive advantage in the field of water electrolysis technology, as well as dominate overseas water electrolysis markets.
Dr. Sung Mook Choi, the principal researcher behind this groundbreaking advancement, expressed his optimism about the future commercialization of anion exchange membrane water electrolysis. Through the development of a one-step hot-pressing electrode fabrication process with high process reliability, one of the key challenges in the commercialization of this technology has been effectively addressed. The future looks bright for green hydrogen production and water electrolysis technology, as Korea continues to innovate and push the boundaries of what is possible in this field.
Leave a Reply