As the world increasingly turns its attention to renewable energy and sustainable technologies, sodium-ion batteries are emerging as a compelling alternative to lithium-ion batteries. With their reliance on sodium—an element vastly more abundant and easier to source than lithium—these next-generation energy storage solutions promise a more sustainable approach to powering everything from electric vehicles to consumer electronics. However, the journey to widespread adoption of sodium-ion batteries presents considerable technological hurdles, most notably the complexities surrounding the preparation of efficient anode materials.
The performance of sodium-ion batteries hinges critically on the anode material used. Unlike lithium ions, sodium ions are larger, necessitating the use of hard carbon instead of the traditional graphite employed in lithium-ion batteries. This is particularly important because hard carbon’s structure supports the larger sodium ions more effectively than graphite. However, extracting and synthesizing hard carbon involves a complex carbonization process where hydrocarbon materials—derived from plants and polymers—must be processed in oxygen-free environments at temperatures often exceeding 1,000°C, which is not only economically burdensome but also poses significant environmental challenges.
This intensive preparation process has rendered the commercialization of sodium-ion batteries difficult, forcing researchers and manufacturers to pursue innovative methods that can streamline production and enhance performance.
Microwave-Induced Innovation
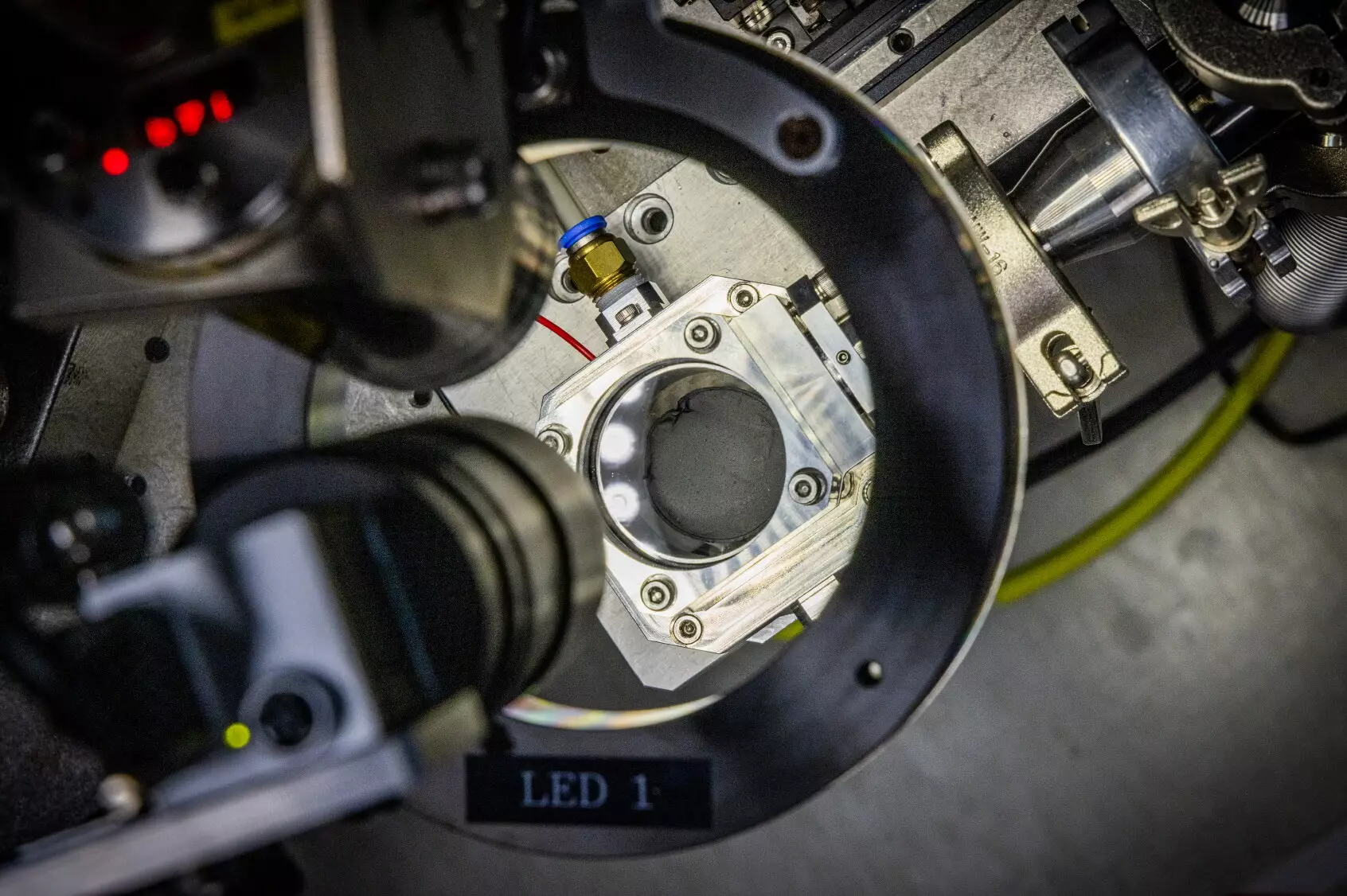
In light of the challenges facing sodium-ion battery technology, an exciting breakthrough has emerged from a research team led by Dr. Daeho Kim and Dr. Jong Hwan Park at the Korea Electrotechnology Research Institute (KERI). This team has pioneered a revolutionary approach to hard carbon anode preparation using microwave induction heating. By leveraging microwave technology—something as commonplace as a kitchen microwave oven—the researchers have developed a method that drastically reduces the carbonization time to merely 30 seconds while achieving temperatures that can exceed 1,400°C.
Their innovative technique involves mixing polymers with conductive carbon nanotubes to form films. When these films are exposed to a microwave magnetic field, electric currents are induced in the carbon nanotubes, resulting in rapid, selective heating. This not only shortens the time frame for preparing hard carbon anodes but also has the potential to reduce overall energy consumption and costs associated with the synthesis process.
What sets this research apart is the application of a multiphysics simulation technique, which grants insights into the intricate processes that occur when electromagnetic fields interact with nanomaterials. This understanding is crucial for optimizing the microwave heating process, allowing the team to fine-tune conditions to yield superior anode materials. The novel methods employed in the study demonstrate the potential of interdisciplinary research, merging principles of engineering, materials science, and simulation technology.
The implications of this breakthrough are significant: not only does it present a pathway to more efficient sodium-ion battery production, but it also opens doors for the technology to be applied in other high-temperature sintering applications, such as all-solid-state batteries.
Despite the promising results, the research team acknowledges that further work remains. They are focused on enhancing the performance of their anode materials and are aiming to develop methodologies that allow for the continuous mass production of large-area hard carbon films. As they advance their technology, there is an expectation that it will attract considerable interest from industries focused on energy storage solutions, paving the way for potential partnerships and technology transfer opportunities.
Dr. Jong Hwan Park has noted the increasing demand for safer battery technologies amidst concerns regarding lithium-ion battery safety, particularly in electric vehicles. With sodium-ion batteries showing promise in colder conditions and exhibiting greater electrochemical stability, the pursuit of commercialization could not be more timely.
In sum, the innovative work being undertaken at KERI marks a pivotal moment for sodium-ion battery technology. By addressing the logistical challenges inherent in the preparation of hard carbon anodes through microwave induction heating, the research team has not only opened new avenues for battery efficiency and sustainability but has also underscored the importance of continuous innovation in the quest for greener energy solutions. As they move forward, the ripple effects of this research may well influence battery technology for years to come, heralding a new era of energy storage that is both efficient and environmentally responsible.
Leave a Reply