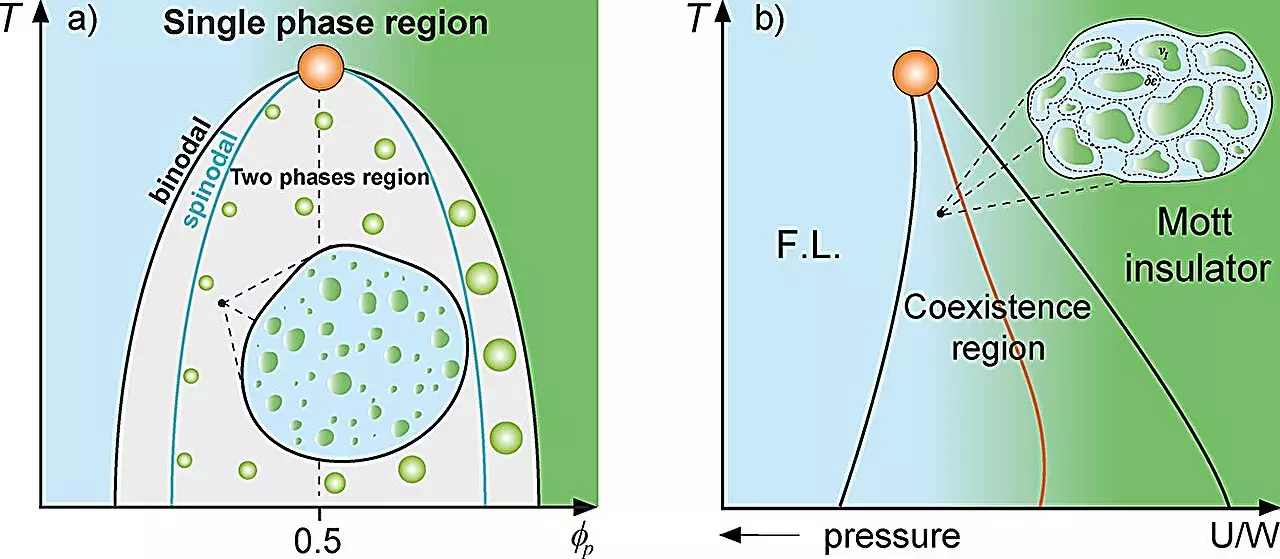
In the realm of physics, particularly within classical mixture theory, scientists often analyze complex systems comprising multiple substances. This theory focuses on the interactions and proportional relationships between different constituents within a mixture. An intriguing application of this theory is seen in physical systems where distinct phases, such as high- and low-density states in supercooled water, coexist. Another compelling example includes the irregular emergence of metallic regions within an insulating matrix, aptly described in the context of the Mott metal-insulator transition. These diverse interactions provide a backdrop against which researchers can explore analogous phenomena in biological systems, specifically in cellular biology.
Recent research conducted by a team at São Paulo State University (UNESP) in Brazil delves into the application of condensed matter physics concepts to understand protein compartmentalization within cells. The study, led by Mariano de Souza and doctoral candidate Lucas Squillante, draws parallels between traditional magnetic Griffiths phases and cellular dynamics, positing the existence of a Griffiths-like phase at the cellular level. The focus is on how specific regions within the cell can experience significant changes in dynamics akin to those observed in a magnetized or paramagnetic context.
Mariano de Souza emphasizes that in magnetic systems, the emergence of regions with differing magnetic properties can dramatically affect the overall dynamics of the system. This understanding sets the stage for investigating how similar “rare regions” of protein droplets impact cellular functions.
A crucial factor in this research is the phenomenon of liquid-liquid phase separation, which occurs when the concentration of proteins within a cell surpasses a certain threshold. This process enables the compartmentalization of proteins into distinct droplets, allowing for specialized functions within the cell. The study employs a range of thermodynamic models, including the Grüneisen parameter and the Avramov-Casalini model, to illustrate that cellular dynamics can be significantly reduced in proximity to the binodal line, a critical parameter that dictates phase separation.
This reduction in dynamics has profound implications for cellular function, particularly relating to the optimization of gene expression. By demonstrating changes in protein diffusion times associated with decreased stochastic fluctuations, the researchers present a compelling argument for how these cellular mechanisms may align with critical events in the origin of life and the evolution of early organisms.
The researchers argue that their findings resonate with historical theories, particularly those proposed by Aleksandr Oparin, who postulated that certain molecular structures, notably coacervates, were vital in the early stages of life. Their slow dynamics likely provided a nurturing environment for the formation and evolution of primitive biological entities. This association raises intriguing questions about the ways in which the physical principles governing protein interactions may have steered the emergence of complex life forms.
Furthermore, the concept of homochirality—where specific molecular orientations dominate in biological systems, such as amino acids—intensifies the discourse. Understanding how the dynamics of protein droplets can influence or even instigate chirality offers a novel dimension to the interplay between physics and biology, suggesting a deeper link to evolutionary processes.
The implications of this research extend beyond theoretical physics or cellular dynamics; they bear significant clinical relevance. The processes associated with liquid-liquid phase separation have been shown to play vital roles in various diseases, including cancer and neurodegenerative disorders. For instance, studies indicate that proteins linked to tumorigenesis may undergo compartmentalization, resulting in altered cellular functions which can predispose to mutations or malignancies.
Marcos Minicucci, a co-author of the study, emphasizes that a nuanced understanding of protein droplet formation can inform therapeutic strategies. Whether these droplets are deleterious or beneficial can significantly influence disease outcomes. The novel Griffiths-like cellular phase proposed by the team could pave new avenues for targeted therapeutic interventions, particularly regarding diseases influenced by protein dynamics.
The study from UNESP underscores the essential role that interdisciplinary approaches play in advancing scientific knowledge. By merging concepts from physics, biology, and medicine, researchers can unlock new insights that can drive innovation in both theoretical and practical applications. As the understanding of protein dynamics deepens, it opens the door to broader implications not only for the study of life’s origins but also for tackling the complexities surrounding modern diseases.
The exploration of a cellular Griffiths phase enriches our understanding of molecular biology by applying physical theories to biological phenomena, promoting a holistic view of life’s processes and their evolution, both in primordial existence and contemporary science.
Leave a Reply