In a groundbreaking development, researchers at the Technical University of Vienna (TU Wien) have achieved a significant milestone in the field of surface chemistry. By generating ion pulses with extremely short durations, well below 500 picoseconds, this innovative technique opens up new avenues for observing rapid chemical reactions in real-time. As we delve into the implications of this research, it’s clear that the synergy between laser technology and ion beams stands to enhance our understanding of material interactions at a molecular level.
In the realm of physics and chemistry, timing is everything. Just as a photographer requires a camera capable of capturing fleeting moments, scientists need advanced tools to monitor ultra-fast processes. Historically, laser pulses have been the preferred method for visualizing atomic processes due to their brief duration. However, the introduction of synchronized ion pulses marks a pivotal development that can complement laser technology. The ability to shoot high-precision ion beams at surfaces in a controlled manner significantly broadens the scope of what can be studied, particularly in the dynamics of chemical reactions.
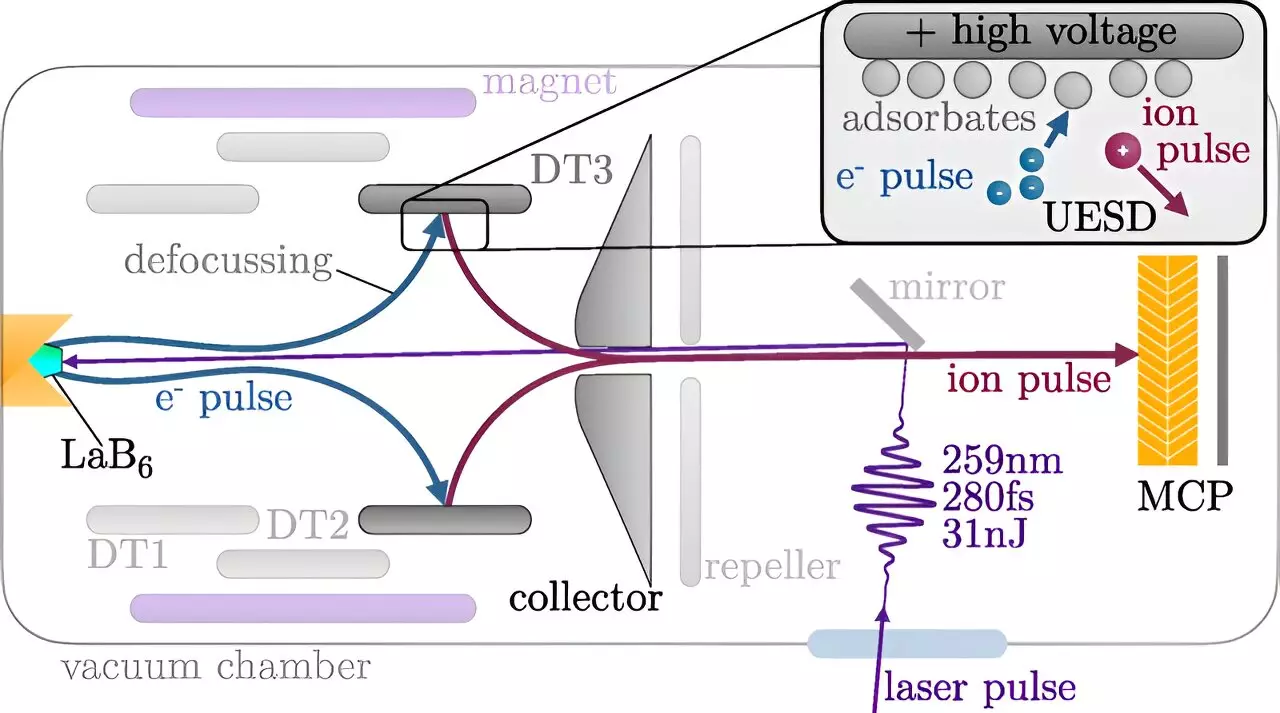
The method established at TU Wien involves a sophisticated, multi-stage process for producing short ion pulses. Initially, a laser pulse strikes a cathode, initiating the emission of electrons. These electrons collide with a stainless steel target, leading to a cascade of events where certain atoms are dislodged from the surface—primarily hydrogen and oxygen. The ability to distinguish between neutral and ionized atoms through electric fields allows scientists to create ion pulses that can be directed at surfaces with precise timing.
Professor Richard Wilhelm notes the novelty of this approach; rather than analyzing the aftermath of ion bombardments, researchers can now probe the real-time impacts of these reactions. By synchronizing the ion pulse with existing chemical processes activated by a laser, they can collect vital data regarding the temporal progression of surface reactions.
The newly developed ion pulses last for less than 500 picoseconds—an astonishingly short timeframe by everyday standards. To give context, this fleeting duration is only a fraction longer than the time it takes light to travel approximately 15 centimeters. While this duration is still longer than the fastest laser pulses, which operate on an attosecond scale, it fits neatly within an optimal range for surface analysis. This precision allows for detailed investigations into the kinetics of chemical reactions that were previously unattainable.
Up until now, protons have been the primary focus for the generated ion pulses. Nonetheless, the technique holds the capability of producing a diverse array of ions, such as carbon or oxygen, depending on the atoms adhered to the stainless steel target. This remarkable versatility enables researchers to tailor their experiments, gaining insights into various aspects of materials science. Furthermore, the generation of electrically neutral atoms and negatively charged ions adds another dimension to this technique’s applicability.
The potential for even shorter ion pulses is already being explored. Researchers anticipate utilizing specifically designed alternating electromagnetic fields to manipulate the speed of ions within the pulse, further reducing their interaction duration. This quest for brevity could not only refine existing methodologies but also revolutionize how ultrafast processes are investigated in both physics and chemistry.
One of the standout features of this technique is its capacity to be intertwined with existing ultrafast electron microscopy technologies. By merging these advanced methods, researchers can gain a multifaceted understanding of surface interactions, yielding unprecedented insights into material sciences. The combination of laser-synchronized ion pulses and electron microscopy paves the way for thorough investigations into the temporal dynamics of chemical reactions.
The research conducted at TU Wien showcases remarkable advancements in the quest to understand rapid chemical reactions on material surfaces. By harnessing the potential of synchronized ion pulses, scientists can probe the intricate processes that govern material interactions, leading to significant implications in both scientific research and practical applications. As this method continues to evolve, it promises to reshape our understanding of chemistry and physics at an atomic level, ultimately influencing future innovations across multiple disciplines.
Leave a Reply