Lithium-metal batteries have the potential to revolutionize the energy storage industry due to their significantly higher energy densities compared to the current lithium-ion battery technology. However, these batteries have been plagued by limitations, particularly in terms of their lifespan. Recent research conducted by scientists at the University of Science and Technology of China and other institutions has introduced a groundbreaking new electrolyte design that could address these limitations and pave the way for the development of high-performance lithium-metal pouch cells with extended lifespans.
One of the primary challenges facing lithium-metal batteries is their limited cycle life, typically around 50 cycles, compared to commercial lithium-ion batteries that can withstand approximately 1,000 cycles. This shortened lifespan is attributed to the growth of lithium dendrites, the reactivity of lithium-metal, and the high-voltage transition metal cathodes, all of which contribute to the degradation of the electrolyte during battery operation.
About five years ago, Professor Shuhong Jiao and her research team developed an electrolyte that stabilizes the interfaces between the electrolyte and the electrodes in lithium-metal battery cells. This innovative electrolyte design aims to suppress the degradation of the electrolyte by simultaneously stabilizing both the anode-electrolyte and cathode-electrolyte interfaces. By leveraging their understanding of the microscopic physicochemical processes inside lithium-metal batteries, the researchers were able to create an electrolyte that could significantly extend the lifespan of these batteries.
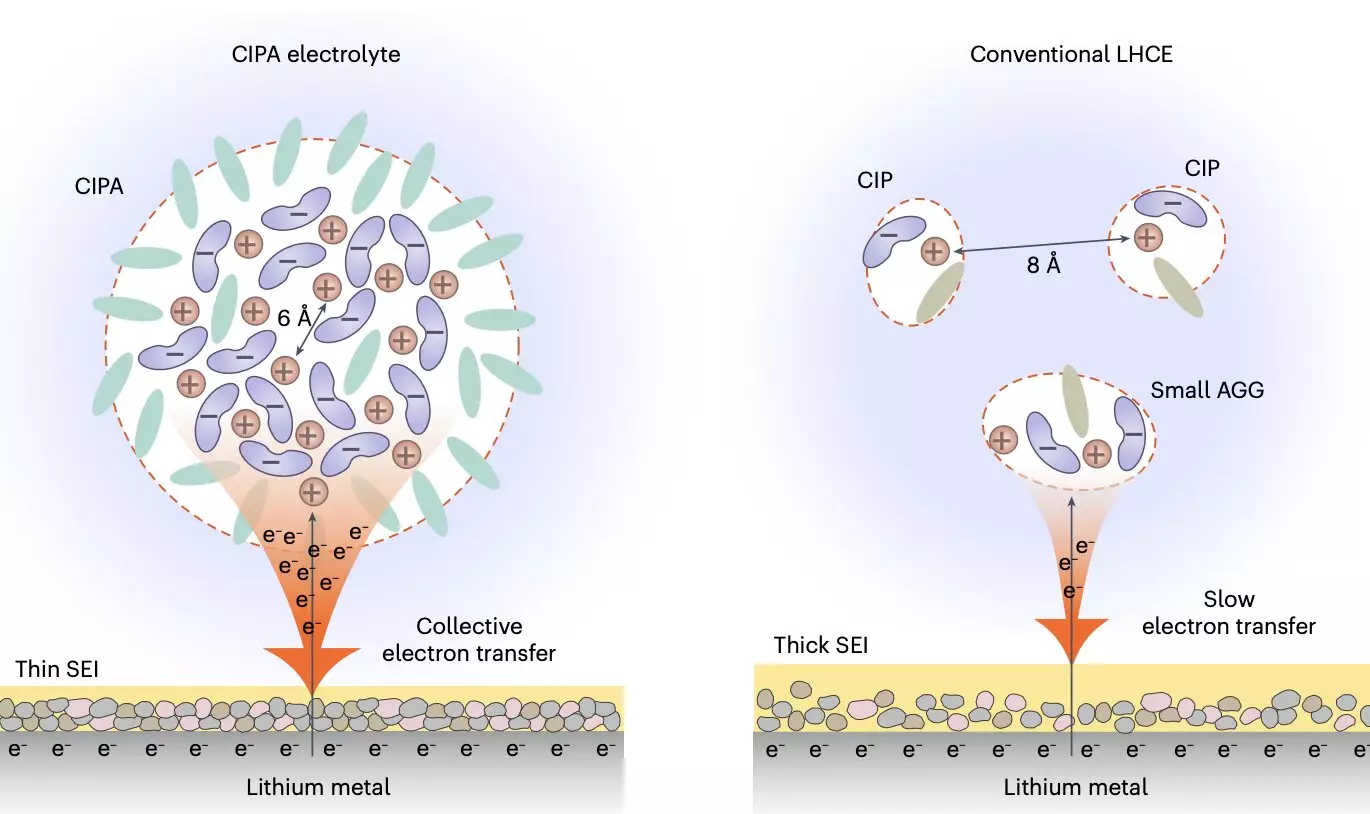
The recent study led by Professor Jiao focused on tuning the solvation structure of the electrolyte at a mesoscopic level, particularly the interaction between ion pairs that influence the formation of the electrolyte’s aggregate structure. The electrolyte designed by the research team features compact ion-pair aggregates (CIPA), which consist of densely packed lithium-anion ion pairs with coordination bonding between them. This unique solvation structure enables the rapid reduction of anions on the surface of the lithium, forming a thin and stable solid electrolyte interface (SEI) that suppresses the degradation of the electrolyte.
The groundbreaking electrolyte design not only promotes a homogeneous and compact lithium deposition but also exhibits good oxidative stability and suppresses the dissolution of transition metal elements from the cathode. This dual effect enhances the stability of both the anode and cathode interfaces, leading to stable cycling performance over a prolonged number of cycles. In initial tests using a 500 Wh/kg lithium-metal pouch cell, the electrolyte retained 91% of its energy after 130 cycles, demonstrating its potential to significantly improve the lifespan of lithium-metal batteries.
The research team’s ultimate goal is to extend the cycle life of 500 Wh/kg lithium-metal pouch cells to more than 1,000 cycles while exploring new battery systems with even higher energy densities and longer lifespans. The innovative electrolyte design introduced in this study has opened up new possibilities for the development of lithium-metal batteries with superior performance characteristics, such as greater energy densities and cycle stability. As researchers around the world continue to investigate and replicate this electrolyte design, the potential for widespread adoption of lithium-metal batteries as the next-generation energy storage solution becomes increasingly promising.
Leave a Reply